To help future parents achieve a successful pregnancy, some clinics offer platelet-rich plasma treatment (PRP) as an additional step during in vitro fertilization (IVF). PRP is made from a small sample of your blood and is rich in natural growth factors. The idea is that these growth factors may improve the uterine lining or even stimulate sluggish ovaries, giving embryos a better chance to implant and grow.
But what is PRP in IVF exactly? This detailed guide explains the procedure and presents the potential benefits and downsides of this treatment.
What is PRP treatment in IVF?
PRP is a concentrated blood product made from your own blood that contains a much higher percentage of platelets than normal blood. Platelets are small cell-like particles that help with clotting and contain natural growth factors. These growth factors play a crucial role in healing, regeneration, reducing inflammation, and stimulating tissue repair.
PRP has been widely used in sports medicine, wound healing, arthritis treatment, anti-aging procedures, and even hair restoration for over two decades. Fertility specialists have begun applying the same idea of using your own growth factors to support reproduction.
In fertility care, PRP is mainly used in two ways:
Endometrial rejuvenation to improve the uterine lining
Ovarian rejuvenation to enhance egg quality and ovarian reserve
Both approaches are still considered experimental, but many clinics offer them as potential options for parents who have had difficulties with standard treatments.

Source: King Shooter
Endometrial rejuvenation: PRP for uterine lining growth
A thick, healthy uterine lining (endometrium) is essential for embryos to implant and grow. But in some women, the lining stays too thin or inflamed, making pregnancy harder to achieve.
PRP is typically done by infused directly into uterine cavity via a catheter to stimulate the progesterone receptors. Here’s what the aim is and when PRP is recommended:
Goal of endometrial rejuvenation | When it’s recommended |
|---|---|
|
|
The procedure is typically performed 48 hours before the embryo transfer. About three to five milliliters of PRP are infused into the uterus, and you can resume your day normally after only 10 minutes’ rest.
If a woman hasn’t been able to develop an adequate endometrium previously, PRP can be performed multiple times: at the initial lining check and 72 hours later if there is no improvement.
Ovarian rejuvenation: PRP for egg quality and ovarian reserve
As women age, the number and quality of eggs decline. That limits the success of IVF, even when using high doses of fertility medications.
Ovarian rejuvenation with PRP can be done in two ways:
Intraovarian PRP: 1–3 mL of PRP is injected into the ovaries using a fine needle guided by ultrasound through the vaginal wall. The procedure is done under anesthesia, and it should be performed one to three months before the start of ovarian stimulation.
High volume intrauterine PRP: 8–10 mL of PRP is infused into the uterus, allowing plasma to reach the fallopian tubes and ovaries. The procedure is done using a catheter with a balloon. Once it’s finished, you will rest for about 10 minutes with the catheter in place.
Here’s what ovarian rejuvenation is intended to do and when it’s recommended:
The goal of ovarian rejuvenation | When it’s recommended |
|---|---|
|
|
How is PRP prepared for fertility treatment?
Blood has four main components:
Plasma: The liquid portion of the blood, mostly water with dissolved salts and proteins. It makes up more than half of the blood volume and serves as a transport medium for other components.
Platelets (thrombocytes): Small particles that help stop bleeding when you’re injured. They also release growth factors that can stimulate tissue repair.
Red blood cells (erythrocytes): The cells that carry oxygen from the lungs to tissues and remove carbon dioxide from the body.
White blood cells (leukocytes): The immune system’s agents, helping protect against infection.

Source: Daniel Duarte
Preparing PRP for IVF is a quick lab procedure. It’s usually prepared from your own blood, even though, in rare nontypical cases, donor blood can be used.
It’s created by concentrating the platelet portion of blood into a small volume of plasma. Here’s the step-by-step process:
Blood collection: The clinician draws a small sample of blood from your vein, similar to what happens during a routine lab test. The volume depends on the amount of PRP required.
Centrifugation: The blood sample is placed into a centrifuge, a machine that spins at very high speeds. The spinning causes the blood to separate into layers based on density. After about 10–15 minutes, red blood cells settle at the bottom, white blood cells form a tiny layer in the middle, and plasma rises to the top. Within the plasma, the platelet-rich layer can be separated from the platelet-poor portion.
Collection of PRP: A lab technician collects the plasma layer, which contains concentrated platelets, and the platelet-rich plasma is then ready for use. Depending on the clinic’s protocol, it may be used immediately or further processed to adjust the platelet concentration.
Is PRP for fertility treatment safe?
Since PRP in most cases comes from your own blood, there’s no risk of transmitted infections, allergic reactions, or rejection, which sometimes happens with donor material. It’s typically considered mostly safe for patients.
Still, no medical procedure is entirely risk-free, so you might experience minor side effects, namely:
Mild discomfort
Cramping
Sensation of pressure during the infusions
Light bruising
Swelling
Soreness at the injection site
These effects are temporary and usually resolve within a few days.
When PRP is injected into the ovaries, it’s done under light sedation and guided by ultrasound. The risk of complications is very low, but there is a slight chance of infection or accidental needle injury to surrounding tissue.
Many women also report some unexpected benefits, such as improved libido and reduced menstrual pain, but these are anecdotal.
When performed in a reputable clinic with strong hygiene standards, PRP is a low-risk, well-tolerated treatment. The main uncertainty lies not in safety, but in how effective it is, since PRP in fertility care is still experimental.
How effective is PRP in IVF?
Researchers are actively investigating whether PRP can have a significant impact on fertility outcomes. While the evidence is still limited and results can vary, early studies are encouraging.
Several studies have looked at women with repeated IVF failures due to a persistently thin or unresponsive uterine lining. One analysis of eight randomized controlled trials involving 678 patients found that women who received PRP infusions experienced significantly better outcomes compared to those who didn’t, namely:
Endometrial thickness increased by an average of 1.23 mm
Clinical pregnancy rate more than doubled
The live birth rate more than doubled
Embryo implantation rate nearly tripled
The cycle cancellation rate was cut almost in half
The study showed that there were no significant differences in spontaneous abortion, chemical pregnancy, or endometrial vascular improvement rates between women who received PRP and those who didn’t.

Source: Cottonbro studio
Studies have also evaluated how PRP improves ovarian function in women with diminished ovarian reserve (DOR). A systematic review and meta-analysis of 38 studies including 2,256 women found that PRP treatment of the ovaries substantially improved multiple fertility parameters, presented below:
Parameter | Findings |
|---|---|
Anti-Müllerian hormone (AMH) | Increase over a period of three months post PRP treatment:
|
Follicle-stimulating hormone (FSH) | Decreased significantly over a period of three months, indicating improved ovarian reserve:
|
Antral follicle count (AFC) | Increased by 1.6 on average, indicating strong ovarian responsiveness |
Number of eggs retrieved | Improved by 0.81 on average |
Number of embryos created | Increased by 0.91 on average |
Spontaneous pregnancy rate | 7% |
Biochemical pregnancy rate | 18% |
Live birth rate | 11% |
Studies show promising results, but reports from actual people who have done PRP as a part of their fertility treatment may be more reassuring:

What else can you do to improve IVF outcomes?
PRP may support ovarian function and uterine health, but improving your IVF chances often requires a comprehensive, multi-faceted approach. Some strategies that can make a real difference are:
Carrier screening for parents: You should determine if you or your partner carry genes for serious genetic disorders that could be passed to your child.
Advanced PGT screening for embryos: PGT-A and PGT-SR screen embryos for chromosomal abnormalities that may impact implantation and healthy development. PGT-M helps identify embryos free from specific genetic conditions known to run in the family.
Polygenic embryo screening: Beyond viability, many parents want to know what the long-term health potential of their embryos is so they can ensure the healthiest start for their future children. Tests like PGT-P and whole-genome sequencing assess multiple genetic factors associated with common chronic and adult-onset diseases, offering predicted health risk estimates.
Personalized support and treatment planning: Age, ovarian reserve, timing of treatment, and individual response play critical roles in IVF success rates.
Nucleus IVF+ combines advanced fertility technologies, expert clinical guidance, and genetic insights into one comprehensive IVF package to help you increase your chances of taking a baby home by up to 20%.

Nucleus IVF+: Where possibilities turn to parenthood
Nucleus IVF+ is the first IVF option that offers genetic optimization with a human touch, helping parents from all walks have the family they have envisioned.
Parents have always done whatever it takes to give their children the best start in life. For many, that means conception through IVF. However, this process also introduces complexity as parents often find themselves juggling the roles of researchers, project managers, and geneticists. Meanwhile, they’re managing the physical and emotional demands of treatment, typically with little guidance.
Nucleus IVF+ changes that, ensuring you never feel alone, from choosing the right clinic to selecting the right embryo and beyond.
Here’s why families choose Nucleus:
24/7 access to expert genetic counselors who guide your decisions and answer your questions
Comprehensive carrier screening for over 2,000 genetic conditions with Nucleus Preview
Access to a network of leading clinics and physicians, chosen for success rates and personalized care
Ability to choose a donor genetically compatible with you
Support for complete IVF cycles, from ovarian stimulation and IVF genetic testing to embryo transfer
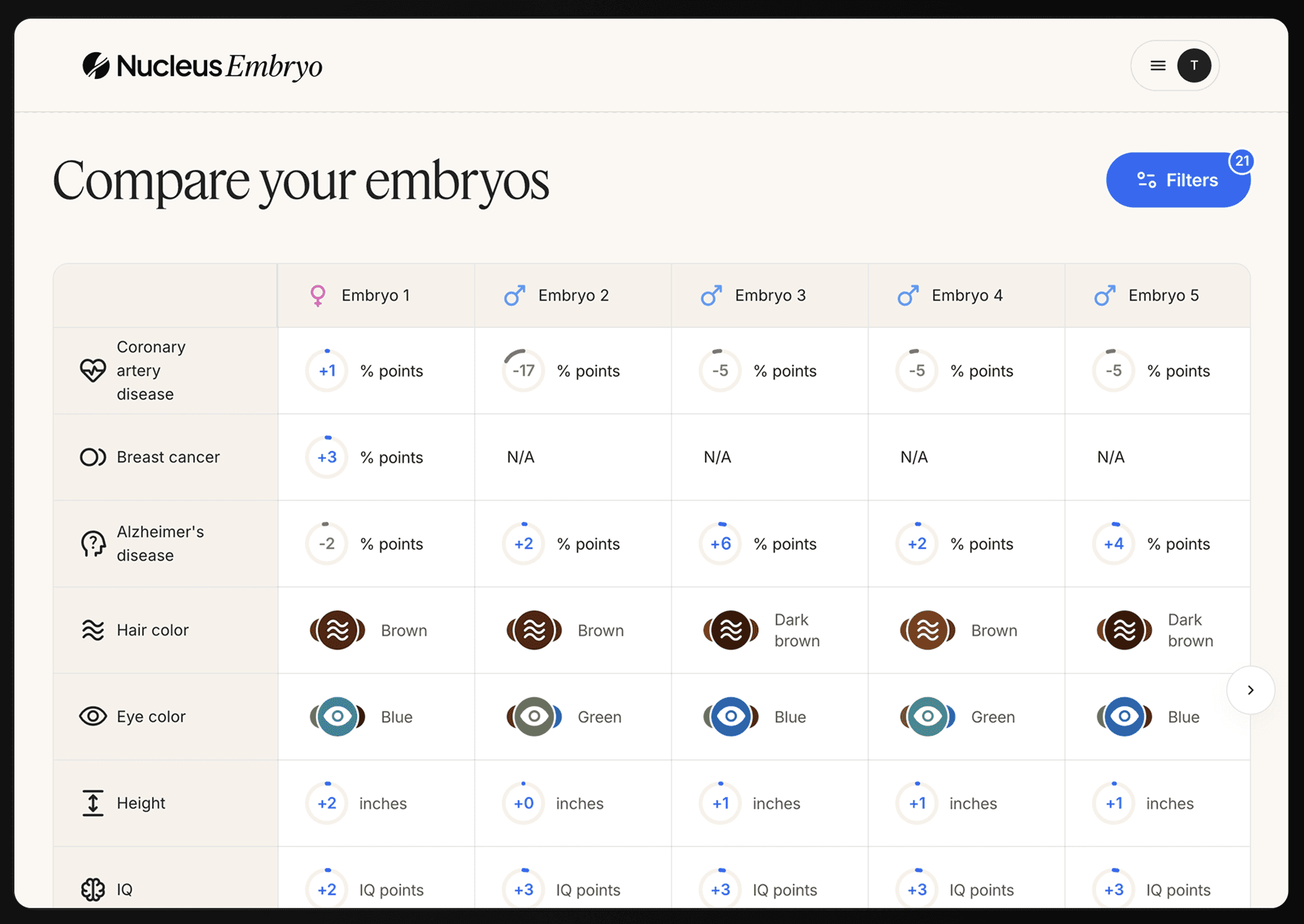
Embryo selection through a genetic optimization software that analyzes your embryos for traits related to health, resilience, and well-being
Actionable health insights for you and your partner with Nucleus Health
If you’re ready to begin your IVF journey, schedule an onboarding call with Nucleus today.
You may also like…
Featured image source: Matilda Wormwood












